
The current position:Home > Information dynamic
> Industry Trends
How to prevent cross contamination of disposable drainage bags
source:m.szkuer.com | Release time:2025年06月30日1、 Product design: Blocking pollution pathways structurally
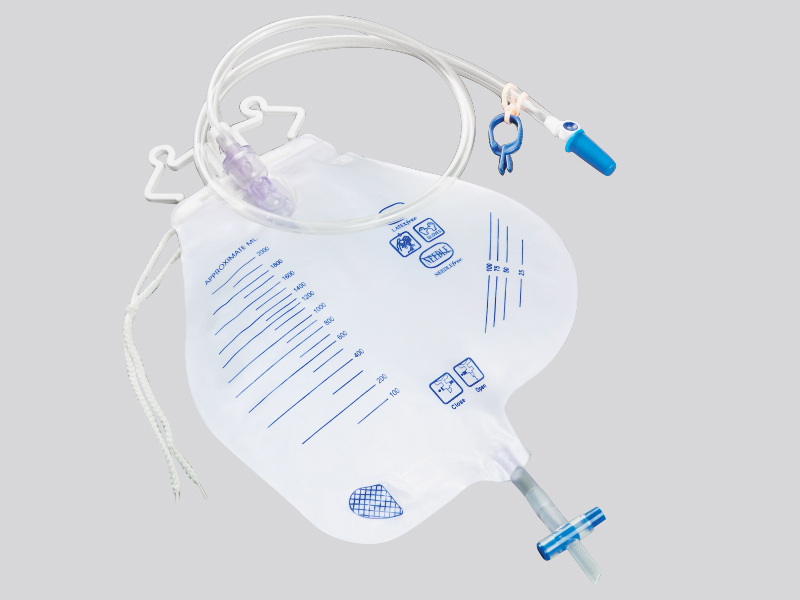
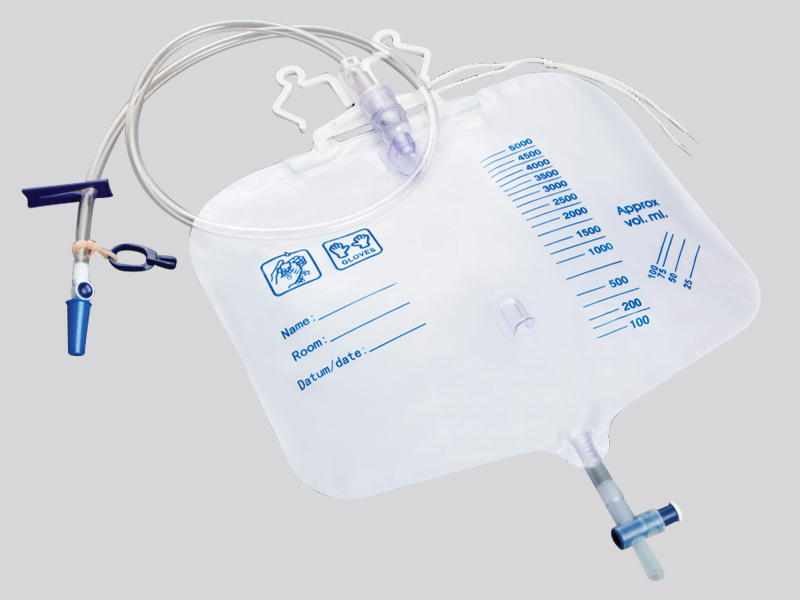
1. Closed pipeline and one-way valve design
Pipeline material and structure:
The main body of the drainage bag is made of medical grade polyethylene (PE) or polyvinyl chloride (PVC), which is non-toxic and resistant to chemical corrosion, avoiding the reaction between the medication and the material to produce pollutants;
The drainage tube is equipped with a one-way silicone valve (such as a duckbill valve or valve structure), which automatically closes when the pressure inside the bag is higher than the outside, preventing the liquid inside the bag from flowing back into the patient's body or the outside air from flowing back. For example, the one-way valve of the chest drainage bag can prevent bacteria outside the chest from entering the chest cavity with negative pressure.
Interface sealing:
The connection between the drainage tube and the puncture needle/catheter adopts a Ruhr lock interface (international standard medical interface), which forms a mechanical seal after tightening, and is matched with a medical grade rubber gasket to prevent liquid leakage and microbial invasion;
Some drainage bags are equipped with sterile protective caps at the interface, which should be removed before use to prevent dust and bacteria from adhering during transportation or storage.
2. Independent packaging and sterilization treatment
Packaging level:
Adopting a dual packaging of "inner bag+outer box": the inner bag is a medical sterilized polyethylene bag, which is sealed after sterilization with ethylene oxide (EO) (sterilization qualification mark: EO sterilization indicator card changes color); The sterile validity period of the outer box printing (usually 2-3 years) is strictly prohibited from use after damage.
Sterilization process control:
The sterilization parameters strictly follow the ISO 11135 standard, with an ethylene oxide concentration of 600-1000mg/L, a temperature of 55 ± 5 ℃, and a sterilization time of ≥ 2 hours. After sterilization, it needs to be analyzed for more than 72 hours to ensure that the residual amount of ethylene oxide is ≤ 10 μ g/g and avoid chemical residue damage to the patient's wound.
2、 Clinical use: Standardized operation to block pollution chain
1. Sterility check before use
Packaging integrity inspection:
Before tearing open the outer box, observe whether there are any signs of compression deformation or moisture damage, and whether the inner bag is bulging (bulging may indicate sterilization failure or packaging damage);
Confirm that the sterilization date is within the expiration date (if it exceeds the expiration date, it needs to be re sterilized or discarded), and record the batch number for traceability.
2. Pollution control during operation
Hand hygiene and sterile gloves:
Medical staff should disinfect with alcohol based hand sanitizer (rubbing time ≥ 20 seconds) or wear sterile gloves before operation to avoid bacterial contact with the interface;
The puncture site needs to be disinfected three times with iodine/alcohol cotton balls in a circular pattern from the inside out, and then connected to a drainage bag after drying.
3. Dynamic monitoring during use
Regular replacement frequency:
Ordinary drainage bags (such as urine drainage) should be replaced every 7 days, and immediately replaced in case of contamination or leakage; Infectious wounds (such as abscess drainage) need to be replaced every 2-3 days to reduce the risk of bacterial growth in the bag (bacteria multiply every 20 minutes in the drainage fluid and can form millions of colonies within 24 hours).
Liquid level and flow rate control:
The capacity of the drainage bag is usually 500-2000ml. When the liquid level reaches 2/3, it should be poured in time. When pouring, the upper end of the drainage tube should be pinched to block the liquid and avoid splashing out and polluting the environment during the pouring process; After pouring, wipe the outer wall of the bag mouth with chlorine containing disinfectant (500mg/L).
3、 Disposal: Cutting off the path of environmental pollution
1. Classification and Sealed Packaging
Identification of infectious medical waste:
The drainage bag after use belongs to "infectious waste" (medical waste classification code 831-001-01) and needs to be placed in a yellow medical waste bag. A "infectious waste" label should be affixed outside the bag, indicating the department, date, and type.
Leak prevention treatment:
After pouring the residual liquid, use specialized scissors to cut open the drainage bag (to prevent repeated use). If the drainage liquid contains blood or body fluids, soak it in 1000mg/L chlorine containing disinfectant for 30 minutes before sealing.
2. Centralized disposal process
Temporary storage and transportation:
The temporary storage room for medical waste must be leak proof, rodent proof, mosquito proof, and fly proof. The temperature should be ≤ 25 ℃ and the storage time should not exceed 48 hours; During transportation, use leak proof turnover boxes and transport them by dedicated personnel and vehicles to the medical waste disposal center.
Harmless treatment:
Using high-temperature steam sterilization (134 ℃, 30 minutes) or chemical disinfection followed by crushing and incineration to ensure pathogen inactivation (such as Escherichia coli and Staphylococcus aureus killing rate ≥ 99.99%), avoiding incomplete incineration and the production of toxic substances such as dioxins.

 Cn
Cn En
En WeChat ID:
WeChat ID: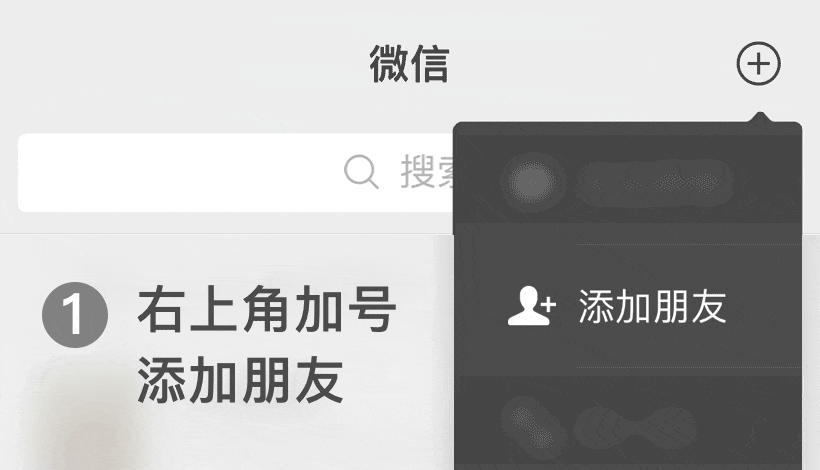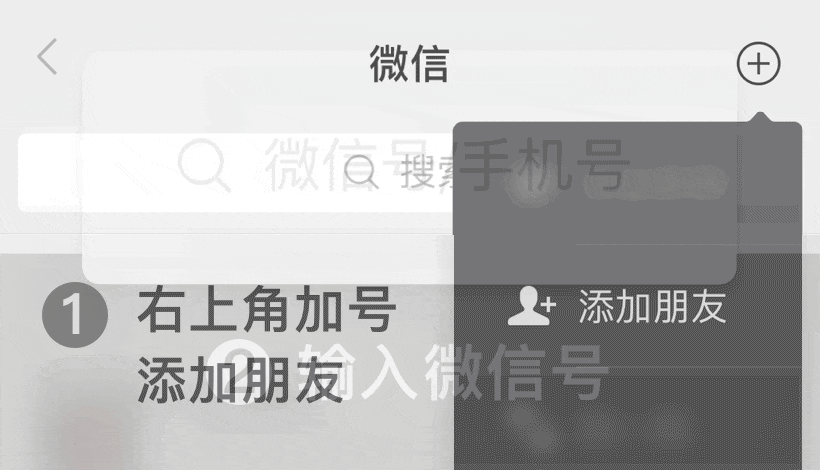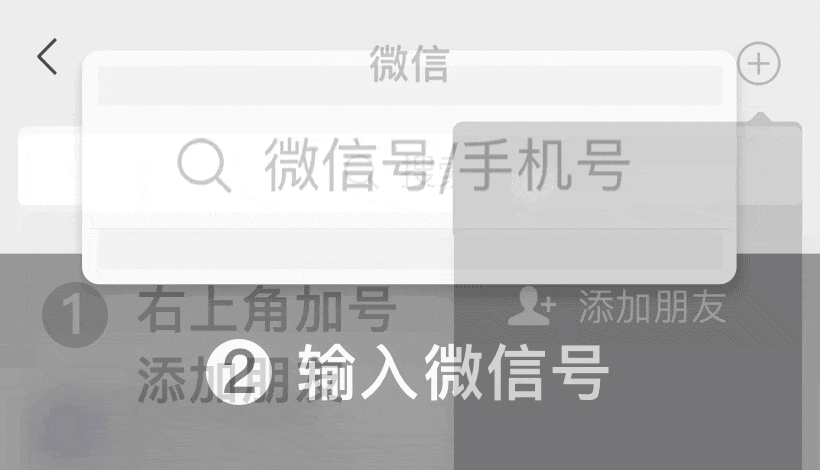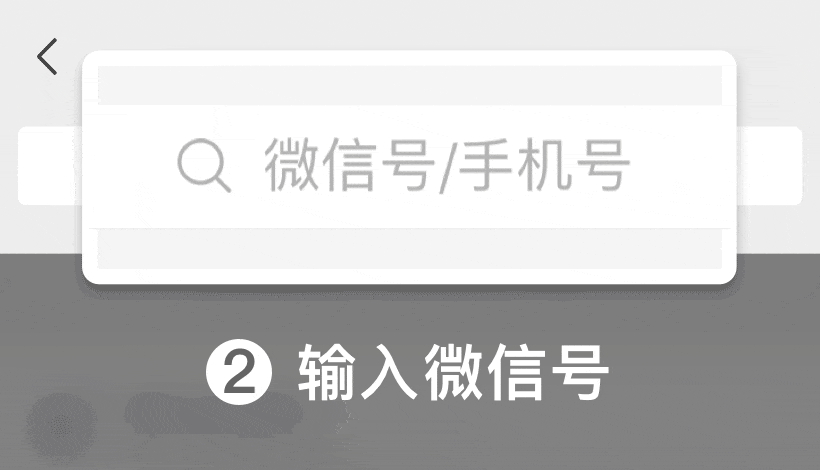




 Contact us
Contact us
 Add WeChat
Add WeChat
 Telephone
Telephone